Chlorofluorocarbons (CFCs) are man-made compounds that consist of carbon, chlorine, and fluorine atoms. They gained popularity due to their stability, non-toxic nature, and low flammability and were used in a variety of industrial and consumer applications, including refrigeration, aerosol sprays, solvents, and foam-blowing agents. CFCs were also used as propellants in aerosol sprays and as blowing agents for foams and packing materials. However, their use has been significantly reduced and prohibited in many countries due to their destructive impact on the ozone layer, referred to as Ozone Depletion Potential (ODP). When CFCs reach the stratosphere, they are broken down by ultraviolet (UV) radiation, releasing chlorine atoms that act as catalysts in destroying ozone molecules, resulting in the thinning of the ozone layer. This ozone depletion has harmful effects, including increased exposure to UV radiation, damage to plant and marine life, and negative impacts on human health. Therefore, it is crucial to study and monitor CFCs to understand their impact on the environment and implement effective measures to reduce their production and use, as outlined in the Montreal Protocol, to mitigate their harmful effects.
| Characteristics | Values |
|---|---|
| Purpose of CFC Study and Monitoring | To understand the impact of CFCs on the environment, specifically the ozone layer |
| Environmental Impact | CFCs contribute to ozone depletion in the upper atmosphere, leading to an increase in harmful UV-B radiation reaching the Earth's surface |
| Ozone Depletion Mechanism | CFCs are broken down by UV radiation in the stratosphere, releasing chlorine atoms that act as catalysts in destroying ozone molecules |
| Health and Environmental Risks | Increased UV-B radiation can cause biological damage to plants and animals, including an increased incidence of skin cancer in humans and genetic damage to many organisms |
| CFC Properties | Non-toxic, non-flammable, stable, and can be readily converted from liquid to gas |
| CFC Applications | Refrigerants, aerosol propellants, solvents, blowing agents for foams and packing materials |
| CFC Phase-out | The manufacture and use of CFCs have been reduced, replaced, or prohibited in many countries due to their environmental impact |
| Montreal Protocol | An international agreement signed by multiple nations to reduce the production of ozone-depleting substances, including CFCs |
What You'll Learn

CFCs' impact on the ozone layer
CFCs, or chlorofluorocarbons, are man-made compounds composed of carbon, chlorine, and fluorine atoms. They are widely used in various industrial and consumer applications, including refrigerants, aerosol propellants, solvents, and foam-blowing agents. CFCs gained popularity due to their stability, non-toxic nature, and low flammability. However, the use of CFCs has been significantly reduced and even prohibited in many countries due to their destructive impact on the ozone layer, known as Ozone Depletion Potential (ODP).
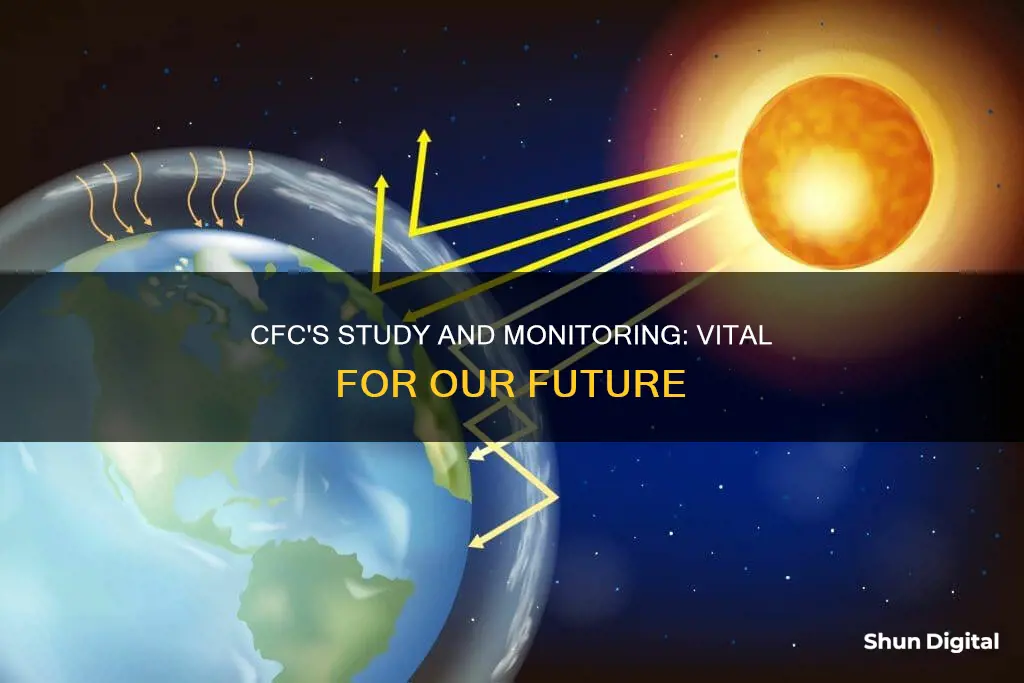
When CFCs are released into the atmosphere, they can reach the stratosphere, where they are broken down by ultraviolet (UV) radiation, releasing chlorine atoms. These chlorine atoms then act as catalysts in destroying ozone molecules, resulting in the thinning of the ozone layer. This process was discovered by University of California chemists, Professor F. Sherwood Rowland and Dr. Mario Molina, in 1974. They found that CFCs could be a major source of inorganic chlorine in the stratosphere following their photolytic decomposition by UV radiation. Some of the released chlorine would then become active in destroying ozone molecules.
Ozone is a trace gas located primarily in the stratosphere, and it plays a crucial role in absorbing harmful UV-B radiation with wavelengths between 280 and 320 nm. This radiation can cause biological damage to plants and animals. Therefore, a loss of stratospheric ozone results in more harmful UV-B radiation reaching the Earth's surface. The impact of CFCs on the ozone layer was significant enough to create a large springtime depletion of stratospheric ozone over Antarctica, observed in the 1980s and worsening each following year. This ozone hole was different from the ozone loss in mid-latitudes due to factors such as the cold temperatures, dynamic isolation, and the synergistic reactions of chlorine and bromine in the Antarctic region.
To address the issue of ozone depletion caused by CFCs, international agreements such as the Montreal Protocol were established. The protocol, signed by 27 nations in 1987, included restrictions on the production of specific CFCs and called for a reduction in their use. Amendments to the protocol in subsequent years further strengthened the call for eliminating CFC production. As a result, CFCs have been replaced by alternative compounds, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs), which have a lower potential for ozone depletion.
While the phase-out of CFCs and the adoption of alternative compounds have helped mitigate their impact on the ozone layer, it is important to continue studying and monitoring CFCs. This is because their atmospheric lifespan can exceed 100 years, and natural and man-made changes can influence the year-to-year variability of stratospheric ozone concentrations. Additionally, there are ongoing issues with CFC smuggling and the continued use of equipment that relies on CFCs, which can pose a threat to the ozone layer and the climate. Therefore, the study and monitoring of CFCs remain crucial to ensure their controlled phase-out and to track the recovery of the ozone layer.
Studio Monitors: Always Sold in Pairs?
You may want to see also

The need for CFC monitoring and control
CFCs, or chlorofluorocarbons, are man-made compounds composed of carbon, chlorine, and fluorine atoms. They gained popularity due to their stability, non-toxic nature, and low flammability, and have been used in various industrial and consumer applications, including refrigerants, aerosol propellants, solvents, and foam-blowing agents. However, the use of CFCs has been significantly reduced and even prohibited in many countries due to their destructive impact on the ozone layer, known as Ozone Depletion Potential (ODP). This impact was first discovered in 1974 by two University of California chemists, Professor F. Sherwood Rowland and Dr. Mario Molina, who found that CFCs, when released into the atmosphere, can reach the stratosphere where they are broken down by UV radiation, releasing chlorine atoms. These chlorine atoms then act as catalysts in destroying ozone molecules, resulting in the thinning of the ozone layer.
The ozone layer is crucial as it shields life on Earth from the harmful effects of the Sun's ultraviolet radiation. Even a relatively small decrease in the stratospheric ozone concentration can result in an increased incidence of skin cancer in humans and genetic damage in many organisms. Due to the growing concern over stratospheric ozone depletion and its dangers, a ban was imposed on the use of CFCs in aerosol-spray dispensers in the late 1970s by the United States, Canada, and Scandinavian countries. This was followed by the Montreal Protocol in 1987, where 27 nations agreed to reduce the production of substances that deplete the ozone layer.
Despite these efforts, the need for controlling CFCs remains urgent. CFCs have a long lifespan, with some lasting over 100 years, and their impact on the ozone layer is cumulative. Natural and man-made changes can influence the year-to-year variability of stratospheric ozone concentrations. Additionally, there are ongoing issues with CFC smuggling, particularly in Asian countries, which undermines the progress made by the Montreal Protocol. Therefore, continuous monitoring and control of CFCs are necessary to minimize their environmental impact and ensure the recovery of the ozone layer.
Effective monitoring requires sensitive and accurate continuous analyzers to measure CFC concentrations, which are currently rare and not readily available. However, organizations such as HORIBA are developing customized CFC analyzers to help address this need. Proper collection, control, and destruction of CFCs are crucial, and preventing the release of these harmful refrigerants is one of the most effective actions to mitigate climate change.
Ankle Monitor Battery Life: How Long Does It Last?
You may want to see also

CFC recycling and recovery
Chlorofluorocarbons (CFCs) are man-made compounds composed of carbon, chlorine, and fluorine atoms. They were once widely used in various industrial and consumer applications, such as refrigerants, aerosol propellants, solvents, and foam-blowing agents. CFCs gained popularity due to their stability, non-toxic nature, and low flammability. However, the release of CFCs into the atmosphere has a detrimental effect on the ozone layer, a phenomenon known as Ozone Depletion Potential (ODP). As a result, the use of CFCs has been significantly reduced and even prohibited in many countries.
The recycling and recovery of CFCs are crucial to minimising their environmental impact. Here are some detailed, direct, and instructive points regarding CFC recycling and recovery:
- Environmental Monitoring and Control: To reduce the environmental impact of CFCs, it is essential to implement efficient control measures during the recycling process. This includes monitoring the concentration of CFCs in the atmosphere and ensuring proper handling and disposal of CFC-containing appliances.
- Sensitive and Accurate Analyzers: There is a demand for sensitive and accurate continuous analyzers to monitor CFC concentrations effectively. These analyzers should be capable of real-time measurements with high sensitivity and customisation options for different types of CFCs.
- Recovery Methods: Various recovery methods are available for capturing and recycling CFCs. One method utilises activated carbon, where the CFCs are adsorbed onto the carbon surface. Once the carbon reaches saturation, it is heated to release the CFCs, which are then condensed and liquefied.
- National Recycling Programs: In some countries, such as the United States, national recycling programs have been established to manage the recovery and recycling of CFC-containing appliances. These programs outline key requirements and prohibited practices to ensure proper handling and disposal.
- Technician Certification: Technicians who service, repair, or dispose of appliances containing CFCs are required to obtain certification from EPA-approved training programs. This ensures that individuals handling CFCs are properly trained and aware of the potential hazards.
- Record-Keeping: It is mandatory to maintain records for servicing, owning, or operating appliances that typically contain more than 50 pounds of refrigerant. These records should include details such as the date and type of service and the quantity of refrigerant added.
- Reporting and Registration: Any release of more than 100 pounds of specific CFCs (e.g., CFC 12 and CFC 113) within a 24-hour period must be reported to the relevant authorities. Additionally, the use or operation of CFC recycling equipment is subject to registration with the EPA.
- Appliance Evacuation: When opening appliances containing CFCs, it is crucial to evacuate the refrigerant to a system receiver or a certified recycling or recovery machine. Evacuation levels are specified in EPA regulations and vary depending on appliance size, usage category, and refrigerant type.
- Smaller Appliance Considerations: For smaller appliances containing less than 5 pounds of refrigerant, such as room-size window air conditioners or residential refrigerators, specific guidelines apply. These appliances can be opened for maintenance or repair without requiring technician certification, but proper disposal practices must still be followed.
- Industry Initiatives: Some companies, such as CFC Recycling, Inc., have taken initiatives to meet community recycling needs. They have established facilities with state-of-the-art equipment for scrap metal, fibre, plastic, and wood recycling.
Easy Speaker Setup: Element Monitor and Desktop Edition
You may want to see also

CFC substitutes
CFCs, or chlorofluorocarbons, are nontoxic and nonflammable chemicals used in aerosol sprays, blowing agents for foams and packing materials, as solvents, and as refrigerants. They are composed of atoms of carbon, chlorine, and fluorine. While they are safe for use in most applications, CFCs can destroy stratospheric ozone.
In 1974, two University of California chemists, Professor F. Sherwood Rowland and Dr. Mario Molina, discovered that CFCs could be a major source of inorganic chlorine in the stratosphere following their photolytic decomposition by UV radiation. This released chlorine, which actively destroys ozone. As a result, more harmful UV-B radiation reaches the Earth's surface, causing biological damage to plants and animals.
In the 1980s, a large depletion of stratospheric ozone was observed, dubbed "the Ozone Hole" by researchers. This ozone hole was particularly prominent over Antarctica due to the region's cold temperatures, dynamic isolation, and synergistic reactions of chlorine and bromine. The need to control CFCs became urgent, leading to the signing of the Montreal Protocol in 1987 by 27 nations. This global environmental treaty aimed to reduce the production of ozone-depleting substances by 50% before the year 2000.
Now, let's discuss the substitutes for CFCs:
The demand for CFCs has been reduced through recycling and the use of alternative substances. Some applications that previously relied on CFCs, such as degreasing metals and cleaning solvents for circuit boards, now use halocarbon-free fluids, water (sometimes as steam), and diluted citric acids. For other uses, industry has developed two main classes of halocarbon substitutes: hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs).
Hydrochlorofluorocarbons (HCFCs):
HCFCs are composed of hydrogen, chlorine, fluorine, and carbon atoms. The presence of hydrogen in HCFCs is advantageous as it reacts with tropospheric hydroxyl (OH), resulting in a shorter atmospheric lifetime compared to CFCs. For example, HCFC-22 (CHClF2) has an atmospheric lifetime of about 12 years, compared to 100 years for CFC-12. However, HCFCs still contain chlorine, so they contribute to stratospheric ozone depletion, albeit to a lesser extent than CFCs. The Montreal Protocol calls for the elimination of HCFC consumption by 2030 for developed countries and by 2040 for developing nations.
Hydrofluorocarbons (HFCs):
HFCs are considered one of the best substitutes for reducing stratospheric ozone loss due to their short lifetime and lack of chlorine and bromine. They do not contribute to the destruction of stratospheric ozone. However, HFCs are efficient greenhouse gases and are targeted for emission reductions in the Kyoto Protocol. For example, HFC-134a (CF3CH2F) has been used in all new domestic automobile air conditioners in the United States since 1993. While HFCs offer lower overall risk than CFCs, the U.S. Environmental Protection Agency (EPA) is concerned that their widespread use could contribute to global warming.
In conclusion, the substitutes for CFCs, namely HCFCs and HFCs, offer safer alternatives in terms of ozone depletion. However, the potential impact of HFCs on global warming is a concern that needs to be addressed. The continuous development and improvement of substitutes are crucial to minimize their environmental impact and contribute to the recovery of the ozone layer.
Monitoring Data Usage: Track Device Consumption
You may want to see also

CFCs' impact on climate change
Chlorofluorocarbons (CFCs) are man-made compounds composed of carbon, chlorine, and fluorine atoms. They are widely used in industrial and consumer applications, including refrigerants, aerosol propellants, solvents, and foam-blowing agents. CFCs gained popularity due to their stability, non-toxic nature, and low flammability. However, their use has been significantly reduced and even prohibited in many countries due to their destructive impact on the ozone layer, known as Ozone Depletion Potential (ODP). CFCs released into the atmosphere can reach the stratosphere, where they are broken down by ultraviolet (UV) radiation, releasing chlorine atoms. These chlorine atoms act as catalysts in destroying ozone molecules, resulting in the thinning of the ozone layer. This depletion of the ozone layer has consequences for climate change.
The ozone layer plays a crucial role in protecting the Earth's surface from harmful UV-B radiation, which can cause biological damage to plants and animals. When CFCs break down in the stratosphere, they release chlorine atoms that catalyze the destruction of ozone molecules, leading to a decrease in stratospheric ozone concentrations. This, in turn, results in more harmful UV-B radiation reaching the Earth's surface. The increased UV-B radiation can have detrimental effects on living organisms, including negative impacts on human health, such as an increased risk of skin cancer and other UV-related issues.
Additionally, CFCs have been identified as potent greenhouse gases, contributing to the "super" greenhouse effect. They have strong absorption bands in the "atmospheric window", a region of relatively high transparency in the atmosphere. The unique susceptibility of the atmosphere at these wavelengths intensifies the "super" greenhouse effect. CFCs have a much higher potential to enhance the greenhouse effect compared to carbon dioxide (CO2) due to their low concentration and linear relationship between mass and atmospheric impact.
The impact of CFCs on climate change has been a subject of scientific debate. While some studies, like the one by Qing-Bin Lu from the University of Waterloo, argue that CFCs are the key driver of global climate change, other researchers have rebutted this claim. Lu's study suggested that CFCs, in conjunction with cosmic rays, caused both the polar ozone hole and global warming. However, critics pointed out flaws in Lu's argument, including the use of outdated data, misinterpretation of previous research, and reliance on unphysical curve fitting. They concluded that the hypothesis blaming CFCs for global warming was inferior to the mainstream explanation and based on fundamentally flawed premises.
Despite the ongoing debate, it is clear that CFCs have had a significant impact on the ozone layer and, consequently, on climate change. International agreements, such as the Montreal Protocol, have been put in place to reduce and eventually eliminate the production and use of CFCs. The success of these efforts is evident in the recovery of the ozone hole and the projected continuation of this positive trend.
Unlocking Monitor Potential: Why is My ASUS Stuck at 60Hz?
You may want to see also
Frequently asked questions
CFCs, or chlorofluorocarbons, are man-made compounds composed of carbon, chlorine, and fluorine atoms. They were once widely used in various industrial and consumer applications, including refrigerants, aerosol propellants, solvents, and foam-blowing agents. However, the release of CFCs into the atmosphere has been found to contribute to ozone depletion, which can result in an increased incidence of skin cancer in humans and genetic damage in many organisms. Therefore, studying CFCs is important to understand their impact on the environment and to develop alternative compounds or technologies that do not contribute to ozone depletion.
Monitoring of CFC concentrations in the atmosphere is important to track the recovery of the ozone layer and ensure that emissions are complying with international agreements and regulations, such as the Montreal Protocol. Monitoring also helps identify any illegal or unregulated use of CFCs, which could hinder the progress made in reducing ozone depletion.
When CFCs are released into the atmosphere, they can reach the stratosphere where they are broken down by ultraviolet (UV) radiation, releasing chlorine atoms. These chlorine atoms act as catalysts in destroying ozone molecules, resulting in the thinning of the ozone layer. The ozone layer is crucial for protecting life on Earth from the harmful effects of UV radiation.
Some alternatives to CFCs include hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs). HCFCs are interim replacements for CFCs as they have a lower ozone depletion potential (ODP) but still contribute to ozone depletion. HFCs, on the other hand, have an ozone depletion potential of zero and are considered one of the best substitutes. Other alternatives include hydrofluoroolefins (HFOs) and natural refrigerants such as hydrocarbons, ammonia, and carbon dioxide.







