Monitoring is a critical aspect of effective management, providing valuable insights for informed decision-making and course correction. It involves the periodic collection, analysis, and utilisation of information to manage performance, maximise positive outcomes, and mitigate potential adverse impacts. Monitoring can be applied to various fields, including education, research, and development sectors. In educational settings, progress monitoring helps teachers evaluate student learning and adjust their instructional methods to improve academic progress. In research, particularly clinical trials, monitoring ensures data integrity and the safety of participants. Additionally, monitoring and evaluation (M&E) are essential in the development sector, where they are used to assess the impact of projects, ensure compliance with regulations, and track financial efficiency.
| Characteristics | Values |
|---|---|
| Purpose | To track the use of inputs and resources, monitor compliance, track the overall setting, track perceptions of beneficiaries, measure financial efficiency, track institutional development, monitor change and progress, and more |
| Timing | Periodic and ongoing |
| Users | Project participants, government department staff, project staff, senior management, fundraising staff, donor organisations, politicians, members of the public, and more |
| Uses | Program management, promoting equity, assessing pilots, identifying problems, compliance and verification, accountability and transparency, ownership and legitimacy, learning, risk management, data for future evaluation, public relations, and more |
What You'll Learn

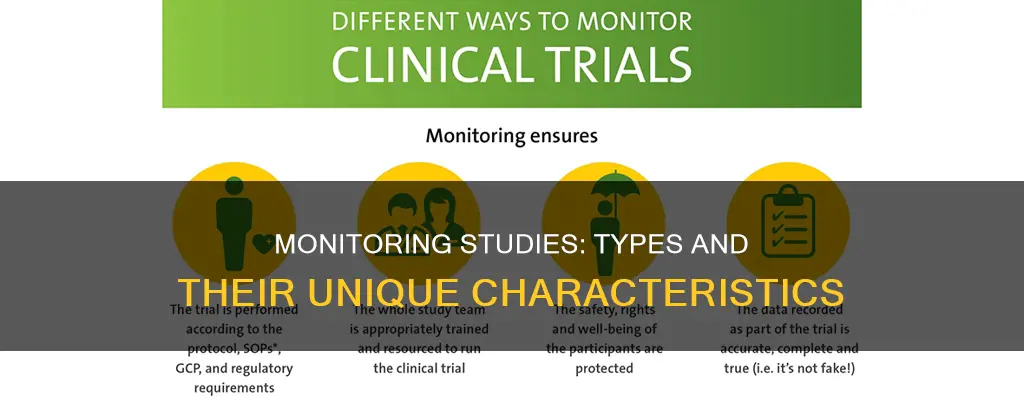
Clinical trial monitoring
Keeping Participants Safe and Respecting Their Rights:
This involves ensuring that participants' safety, well-being, and rights are protected throughout the trial. Monitors must verify that informed consent is obtained, and regularly review safety data and protocol compliance to ensure the protection of participants.
Having Data We Can Trust:
This principle focuses on ensuring data accuracy, completeness, and verifiability from source documents. Monitors implement risk-based monitoring plans, perform on-site and remote monitoring, and conduct audits to ensure data quality and integrity.
Making Sure the Trial is Run as it was Meant to Be:
Monitors ensure compliance with the approved protocol, Good Clinical Practice (GCP), and applicable regulatory requirements. They verify the existence of essential documents, assess site staff training and qualifications, and collect and review protocol deviations to ensure the trial is conducted according to established standards.
- Improving the Way the Trial is Run:
- Preventing Problems Before They Happen:
Monitors develop risk-based monitoring plans, identify critical data and processes, and implement preventive measures to mitigate risks to participant safety and trial processes.
These principles provide a strong foundation for clinical trial monitoring, enabling effective communication and the development of monitoring tools and guidelines.
Monitoring Vyve Data Usage: A Comprehensive Guide
You may want to see also

Data and safety monitoring
Data Safety Monitoring Boards (DSMBs)
DSMBs are typically required for multi-site clinical trials or phase III clinical trials, where the potential risks to participants are higher. The board consists of at least three multidisciplinary members, including physicians, biostatisticians, and other experts such as bioethicists and epidemiologists. They are responsible for interim monitoring of accumulating data to ensure ongoing participant safety, the relevance of the study question, the appropriateness of the study, and the integrity of the data.
Independent Safety Monitors (ISMs)
In some cases, an ISM may be appointed to oversee the monitoring process. This is more common in studies with low-risk interventions, where close monitoring by the Principal Investigator (PI) may be sufficient. The ISM's role is to provide additional oversight and ensure the safety of participants.
Elements of a Data and Safety Monitoring Plan (DSMP)
A comprehensive DSMP should include the following key elements:
- A summary of the protocol, including study design, outcome measures, sample size, target population, and inclusion/exclusion criteria.
- Roles and responsibilities of the monitoring entity, such as the PI, ISM, or DSMB, including their qualifications and monitoring frequency.
- A description of any specific events that would require a participant to discontinue the intervention.
- Procedures for managing medication-related issues, such as allergic reactions or drug interactions.
- Potential risks and measures in place to protect participants, including consent/assent procedures and privacy protections.
- Trial stopping rules, such as increased suicidal ideation or higher-than-expected morbidity rates.
- A plan for managing incidental findings, such as unexpected medical conditions discovered during a scan.
- Mechanisms for addressing and disclosing any conflicts of interest that may impact participant safety or data bias.
- For multi-center studies, procedures for ensuring compliance with the monitoring plan and data reporting across sites.
- Data security measures to protect the confidentiality of the data, such as password-protected encrypted records.
- Processes and timelines for reporting Adverse Events (AEs), Serious Adverse Events (SAEs), and unanticipated problems to appropriate monitoring entities.
- A specific plan and timeframe for reporting IRB actions, protocol violations, or non-compliance issues to the relevant authorities.
- Data management, analysis, and quality assurance procedures, including data source identification and data security measures.
The level and method of monitoring should always be commensurate with the risks involved, the population being studied, and the size and complexity of the trial.
Medical Device Safety Compliance: Who Monitors Usage?
You may want to see also

Process monitoring
Data Collection and Analysis
Checklists and Guidelines
Progress Tracking
An essential aspect of process monitoring is tracking progress against set output targets. This involves establishing output indicators for key activities and setting target values, which can be divided into quarters or years. By tracking progress, project managers can determine whether the project is on schedule and if time-critical activities are being executed as per the calendar.
Feedback and Decision-Making
Continuous Process
M&E, including process monitoring, should be a continuous process integrated into the design stage of a program. It should be conducted at every stage of the project, with data collected, analysed, and utilised on an ongoing basis. This ensures that projects stay on track and facilitates the identification of areas requiring improvement.
Dismantling a ViewSonic LCD Monitor: Step-by-Step Guide
You may want to see also

Compliance monitoring
- Perform a compliance risk assessment to identify potential areas of non-compliance and assess the associated risks.
- Develop a comprehensive compliance policy that establishes clear compliance procedures and is effectively communicated to all members of the company.
- Implement employee training programmes to ensure that every employee understands their role in maintaining organisational compliance.
- Establish monitoring and testing strategies to ensure the effective identification of vulnerabilities and prompt remediation.
- Implement corrective action and remediation plans to address areas of non-compliance and prevent recurrence.
Best Places to Buy Air Register Temperature Monitors
You may want to see also

Context monitoring
The purpose of context monitoring is to provide a comprehensive understanding of the environment in which a project operates. By identifying external factors, context monitoring enables better risk management and informed decision-making. It helps project managers and stakeholders make necessary adjustments to mitigate potential risks and capitalise on opportunities that may arise due to changes in the broader context.
Monitoring Snapshot Removal: Strategies for Effective Surveillance
You may want to see also







